Ethanoic Acid and Sodium Hydroxide
In the informal assessment the learners must determine the temperature change caused by the exothermic reaction of sodium hydroxide and hydrochloric acid. Draw and fully label a titration curve from the data collected from the acetic ethanoic acid and sodium hydroxide titration.
What Is The Reaction Between Acetic Acid And Sodium Hydroxide Quora
For example sodium hydroxide NaOH aq in its aqueous solutions dissociates as.

. Write the balanced chemical equations for the following reactions and identify the type of reaction in each case. Graph the mathrmpH versus mathrmNaOH added. B Fixed bed reactors.
ZnO H 2 SO 4 ZnSO 4 H 2 O. Add 150 mL of M sodium hydroxide solution. Chemical Reagent High Purity Reagent Food Additives manufacturer supplier in China offering Used in Smelting Metal Welding Leather and Dye 995Min Boric Acid Flakes Lmprove Fastness of The Ceramics CAS 10043- 35-3995Min Boric Acid Flakes National Defense and Scientific Research Departments CAS 10043 -35-3 995Min Boric Acid Flakes and so on.
Mass-volume percentage to Molarity Recent user inquiry. To react with a base the amphoteric hydroxide often needs to have been freshly produced and the base must be hot and concentrated. Weak acids and bases are molecules that do not fully dissociate when in solution that is they are not salts.
2022913 1753 Normality to Mass-volume percentage. B Nitrogen tri chloride from ammonia. Repeat the process until the solution has a straw yellow colour.
The health hazards of potassium hydroxide are similar to those of the other strong alkalies such as sodium. STODDARD SOLVENT White spirit. ChlorineI oxide is far less acidic than chlorineVII oxide.
C Concentrated sulphuric acid is added to carbon. Sodium hydroxide NaOH 10 1110. C Sodium chloride from sodium sulphite and dilute hydrochloric acid.
Because these molecules do. Page 6 of 11. Acids Bases and Salts 158 SCIENCE AND TECHNOLOGY Notes MODULE - 2 Matter in our Surroundings Points to ponder All hydrogen containing compounds are not acids Although.
Well take a mixture of ethanoic acid and sodium ethanoate as typical. NaOH HClO 4 NaClO 4 H 2 O. The colour should change from red to brown.
Acetic acid ə ˈ s iː t ɪ k systematically named ethanoic acid ˌ ɛ θ ə ˈ n oʊ ɪ k is an acidic colourless liquid and organic compound with the chemical formula CH 3 COOH also written as CH 3 CO 2 H C 2 H 4 O 2 or HC 2 H 3 O 2. Sour milk Lactic acid. They should understand why the temperature stops changing when all the sodium hydroxide has been neutralised.
Iv Write balanced equation for the following conversions. In the following example zinc oxide becomes the zincate ion ZnOH 4 as part of soluble sodium zincate when added to concentrated base. 2NaOH Cl 2 O 7 2NaClO 4 H 2 O.
A titrimetric method based on the formation of a slightly soluble precipitate is called a precipitation titration. Vinegar Ethanoic acid commonly called acetic acid 3. ChlorineVII oxide itself also reacts with sodium hydroxide solution to give the same product.
An example of a weak acid is acetic acid ethanoic acid and an example of a weak base is ammonia. Mix the solution and leave to stand for one day. A buffer solution has to contain things which will remove any hydrogen ions or hydroxide ions that you might add to it - otherwise the pH will change.
SODIUM HYDROXIDE Caustic soda. If the solution is not decolorized enough add 10 mL of M sodium hydroxide solution. KOH CO 2 KHCO 3.
Vinegar is at least 4 acetic acid by volume making acetic acid the main component of vinegar apart from water and other trace elements. Identify two differences betweeh the two titration cur investigation. Tubular reactors are used for example in the steam cracking of ethane propane and butane and naphtha to produce alkenes.
3 a Lead sulphate from lead nitrate and sulphuric acid. SULFURIC ACID concentrated 51 and 100 Sulphuric acid. ChloricVII acid reacts with sodium hydroxide solution to form a solution of sodium chlorateVII.
A heterogeneous catalyst is used frequently in industry where gases flow through a solid catalyst which is often in the form of small pellets to increase the surface areaIt is often described as a fixed bed of catalyst Figure 5. A typical resin is that produced from a polyol such as propane-123-triol glycerol with a dibasic acid such as benzene-12-dicarboxylic phthalic anhydride and a drying oil linseed or soybean oil. 2-ETHOXYETHYL ACETATE Ethylene glycol monoethyl ether acetate.
The most important precipitation process in titrimetric analysis utilizes. ACETIC ACID Glacial acetic acid. Some 3d metal compounds such as chromium hydroxide chromiumIII oxide ferric oxide has amphoteric characteristics.
C Ethanol is warmed with ethanoic acid to form ethyl acetate in. Acetone acetonitrile ACN benzene ethanol diethyl ether isopropanol methanol methyl ethyl ketone tetrahydrofuran THF. Ethanoic acid CH3COOH 36 1045 hydrochloric acid HCl 36 1180 nitric acid HNO3 67 1400.
Identify any buffer regions on your titration curve. H 2 SO 4. A Nitrogen gas is treated with hydrogen gas in the presence of a catalyst at 773K to form ammonia gas.
Ethanoic acid is a weak acid and the position of this equilibrium. When dealing with a strong acid and a weak base or vice versa the titration curve becomes more irregular. Amphoteric characteristics of chromium compounds.
The addition of hydroxide ions by adding lime sodium hydroxide or potassium hydroxide adjusts the pH because the hydroxide ion reacts with carbon dioxide to form bicarbonate alkalinity. Tamarind T artaric acid 4. Health Hazards of KOH.
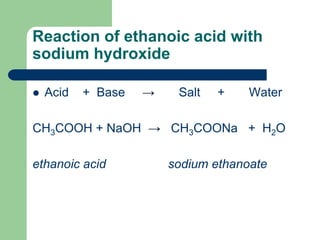
Decorative gloss paints typically contain alkyd polymers resins. B Sodium hydroxide solution is treated with acetic acid to form sodium acetate and water. Mix the solution and leave to stand for one day.
Amphoteric properties of chromium hydroxide CrOH 3 Chromium hydroxide CrOH 3 is an amphoteric compound and a green precipitateWhen NaOH aq is added that precipitate. On being heated together ester linkages are formed and water is a by. Acidic and alkaline buffer solutions achieve this in different ways.
2022913 1708 Molarity to Molarity.

Net Ionic Equation For Naoh Ch3cooh Strong Base And Weak Acid
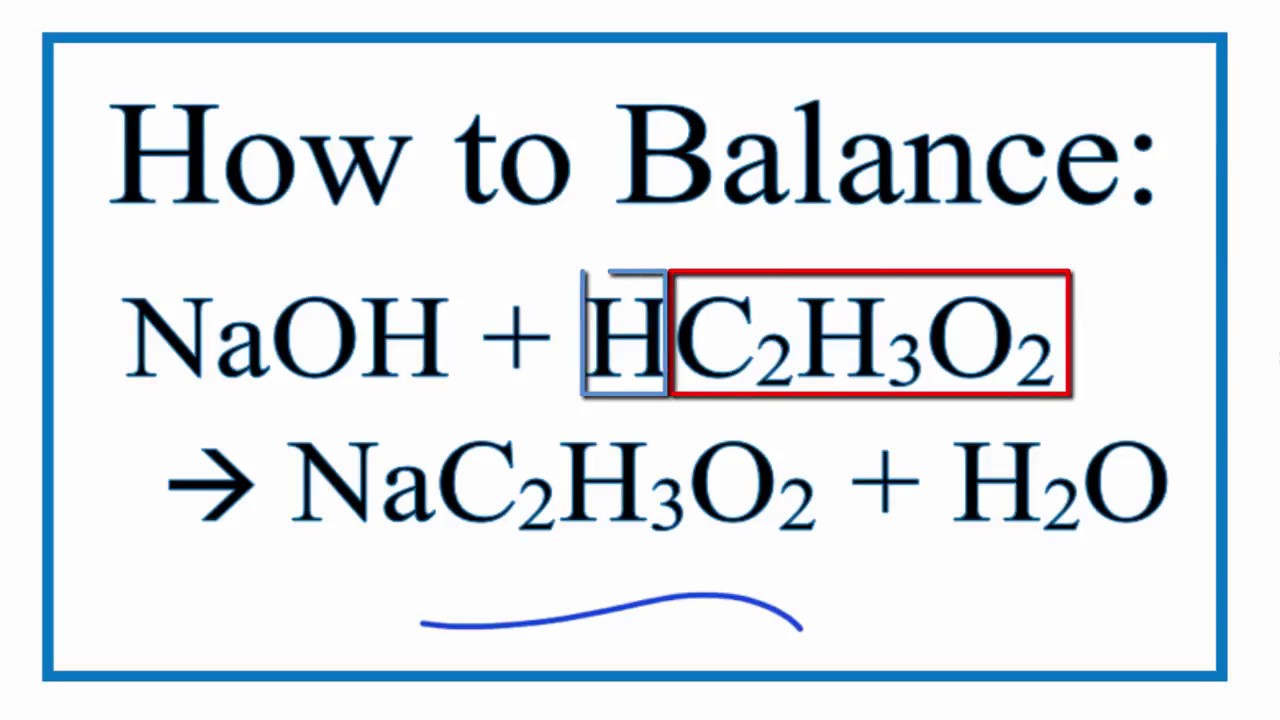
How To Balance Naoh Hc2h3o2 Nac2h3o2 H2o Sodium Hydroxide Plus Acetic Acid Youtube
No comments for "Ethanoic Acid and Sodium Hydroxide"
Post a Comment